
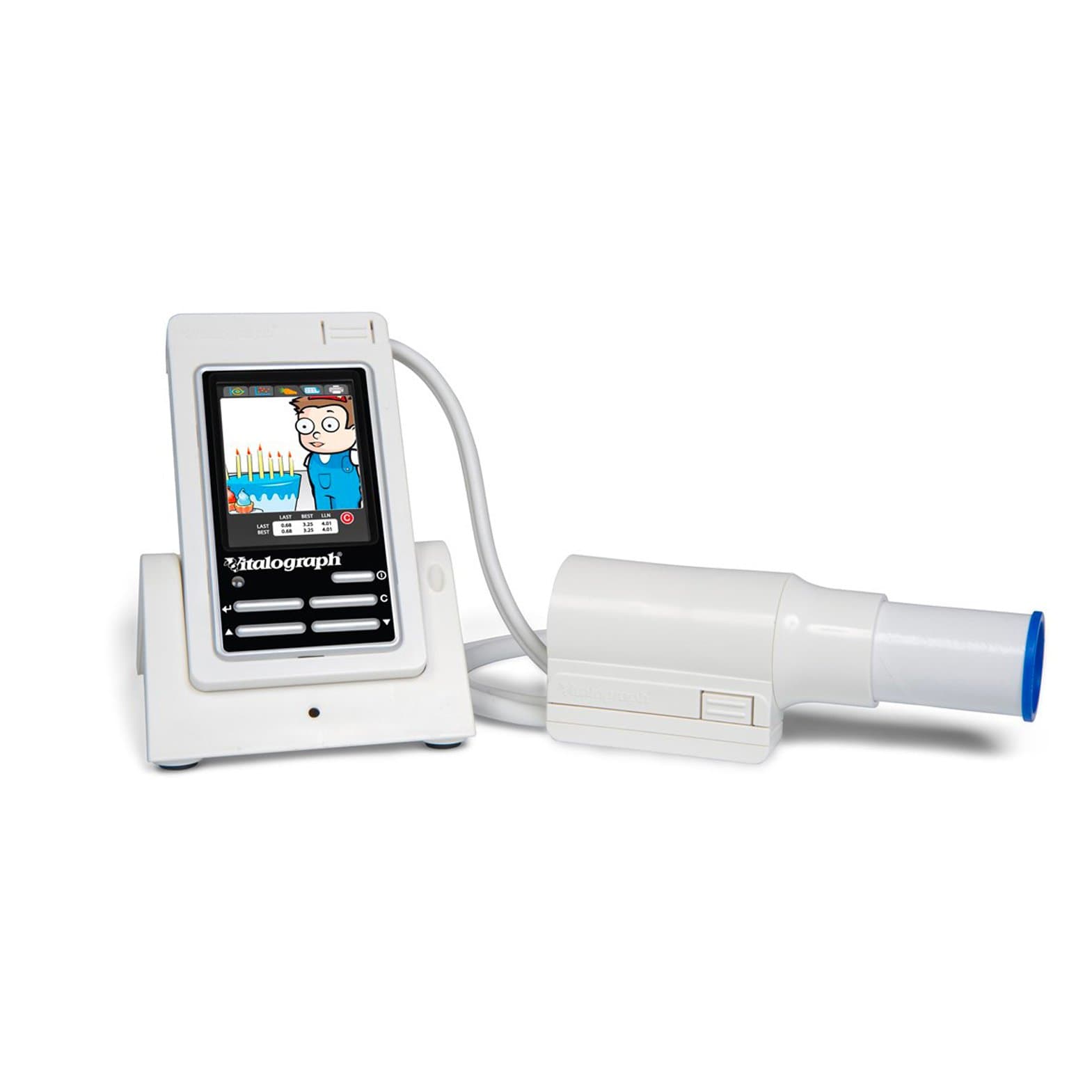


Vitalograph In2Itive Hand Held Spirometer
Vitalograph In2Itive Hand Held Spirometer
In2itive Spirometer
High quality mobile spirometry with PC & network connectivity
Vitalograph In2itive™ is an easy to use, lightweight handheld spirometer that combines the benefits of portability with the power of a networkable PC spirometer.
The precise and durable measurement technology is extremely accurate and stable over time, assured through our simple calibration check routines as recommended by international spirometry guidelines (ARTP, ATS/ERS, etc.) and good practice. No need for expensive disposable sensors, turbine, spirettes nor flow tubes with optimal hygiene assured via Vitalograph Bacterial Viral Filters (BVF™).
A high resolution colour touch screen display, combined with an intuitive icon driven user interface, makes the In2itive quick and simple to operate. A database for over 10,000 patients, child friendly incentive animations and the latest GLI spirometry predicted equations and Z-scores contribute to all round suitability for adult and paediatric patients within general healthcare and occupational health environments.
The In2itive is supplied with Vitalograph Reports PC Software with the ability to create editable PDFs for filing and manual upload to electronic medical record (EMR) systems.
Customers can also choose to upgrade to the In2itive with Spirotrac® Software, which allows for automatic data exchange, via HL7 and GDT transfer protocols, with EMR systems and the ability to add other diagnostic tests and physiological measurements (including resting 12-lead ECG, SpO2, NIBP and Audiometry).
Includes:
- EMIS*
- Vision*
- Cohort
- Medgate
- SystmOne*
- Clinic-All
- eOPAS
- Cradle for battery charging, connection to a PC and automatic data exchange saving time and eliminating errors.
- Assured hygiene with removable flowhead and Vitalograph Bacterial Viral Filters (BVF)
- Hours of battery life for a full day of off-site testing
- Over 50 test parameters
- Pre-post bronchodilator comparison
- Single and multi-breath
- F/V or V/T curves viewable in real time
- Choice of child incentives with sound effects
- Remote flowhead option enables the subject to view incentives while performing the test
- Icon driven menus
- Print via your PC or via Spirotrac Software or print direct to a USB A4/Letter size printer using optional printer cradle
- Range of accessories and robust carrying case
| Product Name: | In2itive Handheld Spirometer |
| Model No: | 2120 |
| Subjects: | Automatic population of database from Spirotrac. Name, ID; Age, Height, Gender; Smoking status, Body Mass Index and more depending on model. Storage of up to 10,000 subjects. |
| Environmental Data: Selectable: | Temperature (built in sensors), Barometric pressure, altitude, humidity. |
| Test types: | Single breath tests, flow/volume loops, multi-breath testing, tidal breathing and combined VC/FVC type test methods supported |
| Selectable Parameters (depending on model): | VC; IVC; IC; VT (TV); TLC; RV; IRV; ERV; FRC; FVC; FIVC; FEV1; FEV3; FEV6; FVC; FEV1/VC; FEV1/FVC; FEV3/VC; FEV3/FVC; FEV1/FEV6; FEF75; FEF50; FEF25; FEF25-75; FEF25-75/FVC; FIV1; PIF, FIV1/ FIVC, FIF25, FIF50, FIF75, FEF50/FIF50, FET, MVVind, FEV1 Ratio; FEV0.5; PEF L/min; PEF L/s; FEF 0.2-1.2; FEF 75-85%; FEF25%; FEF50%; FEF75%; FMFT; FIF25%; FIF50%; FIF75%; PIF; FEV0.75; Lung Age and more. |
| Predicted Values Selectable(depending on model): | ERS/ECCS; GLI, NHANES, Polgar, Pereira, Berglund,Forsche, Gutierrez,Hedenström, Taiwan;Knudson, Crapo; Hsu; KNLW; Viljanen; SEPAR; Gulsvik; SBPT; Tamura; Ip; Quanjer; Wang; Dockery and more |
| Suggested Interpretation: | User selectable |
| Flow Technology: | Fleisch Pneumotachograph (No3 size) |
| Resolution: | 10 mL volume; 0.01 L/s flow |
| Data Storage: | Stores up to 10,000 subjects and hundreds of test sessions |
| Accuracy when in Operating Range: | Volumes: Better than ± 3% (Max 10L / Min 0L) Flows: Better than ± 10% (Max 16L/s / Min 0.02L/s) Linearity: ± 5% in range 0.1 L/s to 16 L/s |
| PowerSAFE™: | Input 100 - 240V AC 50-60Hz, output 5V DC |
| Battery Pack: | Rechargeable Lithium Polymer 3.7v 2000mAH |
| Dimensions: Device: | 160mm x 100mm x 45mm (with flowhead attached) |
| Nett Weight: | Device: 230g |
| Gross Weight and Size (Packed): | Weight: 1.5kg, Packed Size: 213mm x 199mm x 158mm |
| Storage Humidity: | 10% to 95% |
| Storage Temperature: | 0°C to 50°C |
| Recommended Operating Temperature Range: | 17 - 37°C; Design Limits 10-40°C |
| Ambient Humidity: | 0% to 99% |
| Connectivity: | USB 2 |
| Max Test Duration: | 20s FVC; 30s VC |
| Performance Standards: | ISO 26782:2009 ISO 23747:2007 ATS/ERS 2005 |
| Safety Standards: | IEC 60601-1:2005 |
| Medical Safety Standard: | Medical Devices Directive 2007/47/EC |
| Designed & Manufactured Under: | ISO 13485:2003 and 2016 FDA 21 CFR 820 CMDR |




